The European Commissioner for Health and Food Safety Stella Kyriakides stated in her keynote speech (Continuing the Conversation on the Digital Transformation of Healthcare) this year what the European Health Data Space (EHDS) will be about, for both patients and research. Because I believe the EHDS will have a big impact on our healthcare ecosystem, I decided to write this post, to give you a better understanding on what exactly the EHDS is, explore the main topics, rights and responsibilities, and what is already being done today to make this a reality.
The EHDS overall objectives are the following:
- Empower individuals to control their health data
- Foster a single European market for digital health services and products
- Ensure interoperability and security of health data and a level playing field for manufacturers
- Unleash the power of the health data economy
- Ensure a consistent and efficient framework for the reuse of health data for research, innovation, policy-making and regulatory activities
Patients
- For patients to have the right to use and access their electronic health data in a format that can be accepted across the EU, for free.
- For patients to be able to share data with a health professional of their choice, even across borders.
- For patients to be in control of their own data, to be able to restrict what part of their medical history is shared with whom, or to correct any errors in their health records.
- Ensure transparency so patients can see who has accessed their data.
Research and Innovation
- Access to health data in an interoperable, digital format for research and innovation will require time bound permits that clearly spell out the specific purpose.
- Access will be possible only to data that does not reveal the identity of individuals in the dataset.
- The data will be accessed in closed and secure environments, and only anonymized data will be downloadable.
But what does this mean for our European healthcare landscape and what will be needed to achieve this initiative? At a high level we can divide the EHDS in 2 main blocks: Primary and Secondary use of health data, combined with new rules on electronic health record vendors. Where Primary is mainly focused on the patient side and Secondary on the Research and Innovation side.
Primary use versus Secondary use of data
MyHealth@EU
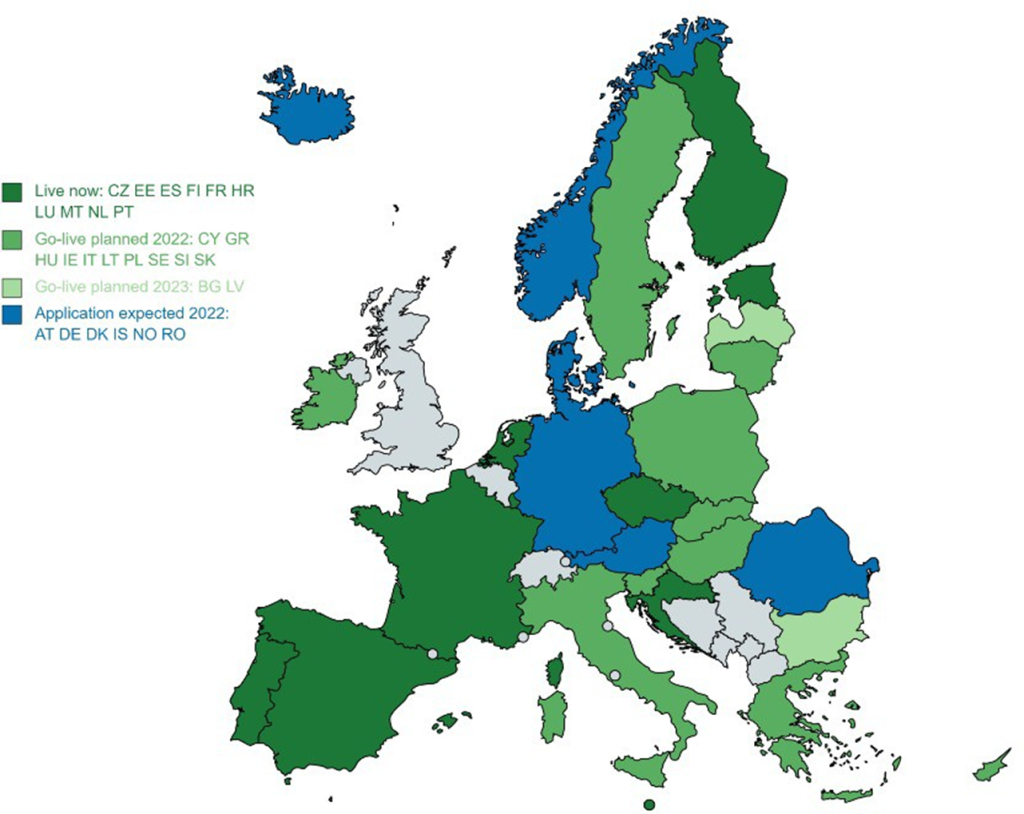
When talking about primary use of data within the EU, we talk about MyHealth@EU. This EU initiative is to support patients to exchange health data cross border. Currently there are 10 Member States live and the commission expects the number of connected Member States to grow in the coming years. There are 2 services available: Patient Summary and ePrescription. These services will be expanded to also include medical images, Lab results, Discharge letters, rare disease data and other health information categories.
Secondary use in the EHDS
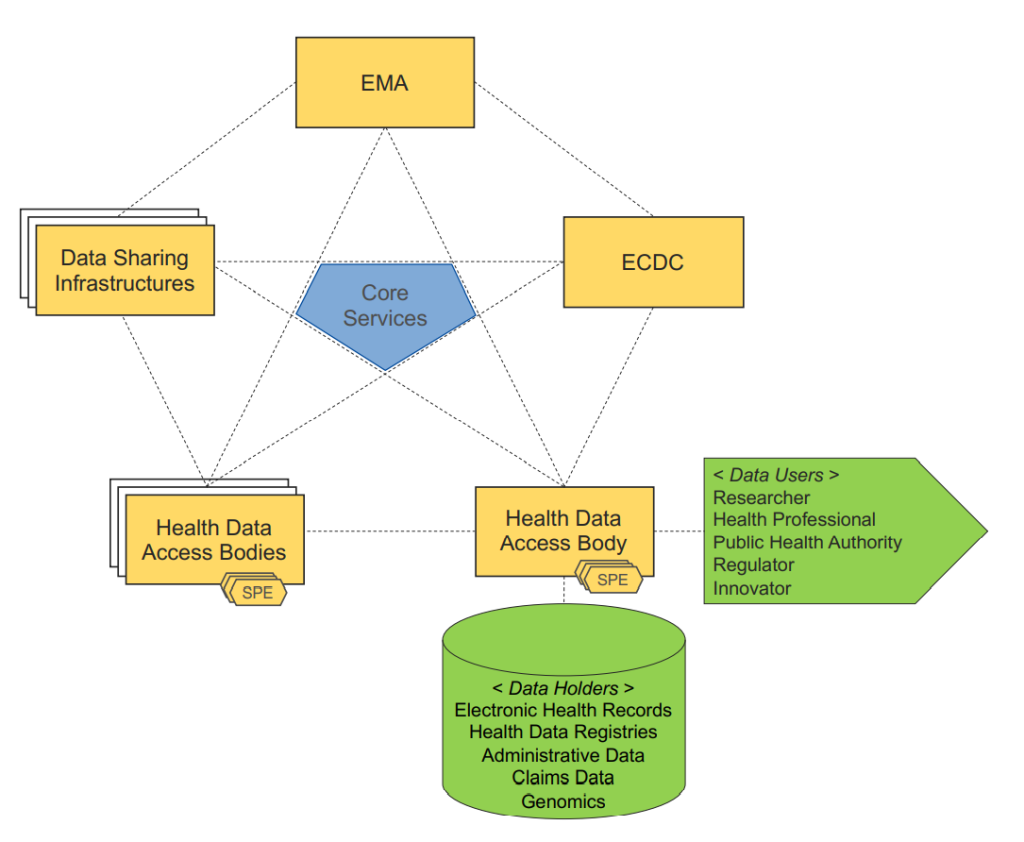
To efficiently enable re-use of healthcare data by researchers, policymakers and industry, the EU is looking to create a network of nodes as entry points into the EHDS. These nodes would be the National Data Authorization Bodies complemented with European stakeholders, such as the European Medicine Agency (EMA), European Centre for Disease Prevention and Control (ECDC), or other authorized data holders. An important factor here will be the interoperability of digital healthcare data, combined with the quality, privacy and security of this healthcare data. All data for secondary use will be de-identifiable data. There will be a focus on a number of areas, such as expanding the existing infrastructure in Member States combined with an introduction of an European infrastructure.
New rights and responsibilities to use and access patient data in the EU
With the creation of the EHDS, there will be new rights for patients and health professionals, combined with new responsibilities for electronic health records vendors and member states.
- New patient rights: Patients will have access to their electronic health data, immediately, free of charge and in an easily readable, consolidated and accessible form and use an electronic health data access service to access that data. The member States will be required to establish one or more electronic health data access services at national, regional or local level.
- New abilities for the health professionals: health professionals will get the ability to access the electronic health data of individuals under their treatment, irrespective of the Member State of affiliation and the Member State of treatment. And in order to support cross-border continuity of care, the draft EHDS Regulation requires Member States to designate national contact points for primary use of electronic health data
- Specific rules related to electronic health record systems: The draft Regulation imposes obligations on manufacturers placing EHR systems on the market in the EU, including requirements in relation to security and interoperability.
- Secondary use of electronic health data: Data holders are a defined category of private entities and public bodies in the health or care sector performing research in relation to these sectors. They will be required to make certain health data available for specified secondary use cases. To access this data, data users must obtain a data permit from a health data access body in the relevant Member State or from the data holder.
- National governance: Each Member State will be required to designate a digital health authority, responsible for undertaking a range of obligations the draft Regulation. Health data access bodies are tasked with monitoring and supervising compliance by data users and data holders with the rules on secondary use of electronic health data. The Commission will also establish a “European Health Data Space Board as an advisory committee and expert group. The Board will be made up of representatives of Member State digital health authorities and health data access bodies, and the Commission.
Towards European Health Data Space
Towards European Health Data Space or TEHDAS, is a Joint Action project being carried out by stakeholders from 25 European countries. Their goal is to develop European principles for the secondary use of health data and to advance the cross-border secondary use of health data in Europe.
TEHDAS is doing this by structuring their work in 8 different work packages.

Every couple of months there is an update on one of the work packages. Such as the result of consulting stakeholders on data altruism, suggesting options to overcome data barriers, identifying funding options for secondary use of health data, … Below you can find a snippet on one of the reports regarding potential barriers in on secondary use of health data.
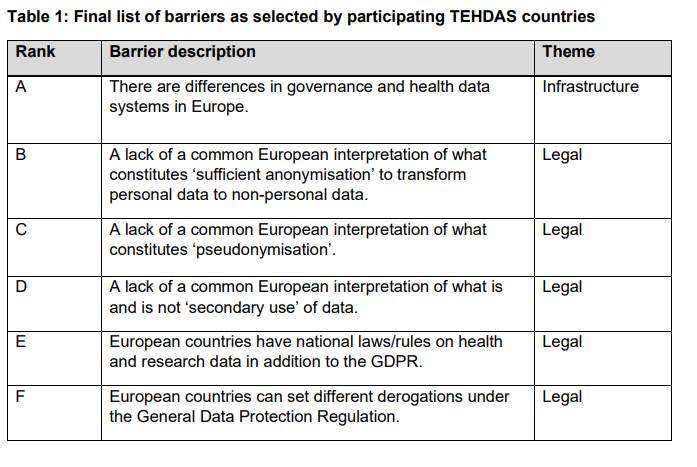
data
Existing pilots and consortia
There are currently several projects and consortia approved which will start piloting and impacting the EHDS. Such as the Data Analysis and Real-World Interrogation Network (DARWIN EU®), orchestrated by EMA. Or the new EHDS consortium led by the French Health Data Hub, which will feed the legislative discussions around the draft regulation proposed by the European Commission.
Data Analysis and Real-World Interrogation Network (DARWIN EU®).
The European Medicines Agency (EMA) is establishing a coordination center to provide timely and reliable evidence on the use, safety and effectiveness of medicines for human use, including vaccines, from real world healthcare databases across the European Union (EU). This capability is called the Data Analysis and Real-World Interrogation Network (DARWIN EU®). DARWIN EU will deliver real-world evidence from across Europe on diseases, populations and the uses and performance of medicines.
This project will connect the European medicines regulatory network to the European Commission’s European Health Data Space (EHDS), an initiative to promote better exchange and access to different types of health data. While the needs and use cases of medicine regulators and decision-makers will drive DARWIN EU’s development, DARWIN EU will also contribute to developing the EHDS and the joint action to deliver European principles for the secondary use of health data, known as Towards European Health Data Space (TEHDAS).
Acting as an early flagship ‘pathfinder’ for the EHDS, DARWIN EU will enable the exchange of healthcare data for use in healthcare delivery, policy-making and research across Europe, while fully complying with data protection requirements.
EHDS consortium led by the French Health Data Hub.
Originated from an EU4Health call, the European Commission has announced its decision to choose the consortium led by the French Health Data Hub, to set up a pilot project for the European Health Data Space. This project will aim to feed the legislative discussions around the draft regulation proposed by the European Commission on May 3rd. The consortium which consists out of sixteen partners, from ten European countries, such as FINDATA, Danish Health Data Authority, Norwegian Directorate of eHealth, Sciensano, … will have the objective to address the challenges surrounding access to health data throughout the EU and to open new perspectives for research and innovation.
Summary
The EHDS will have a significant impact on patients, healthcare providers, payers, pharma and Member States and how we will work with healthcare data in the future. We already see several Member States starting or extending their healthcare data strategy, to be ready for the EHDS in 2030. And there are already multiple workstreams ongoing, such as:
- legislative and policy discussions: These discussions are now mainly through TEHDAS and validation through consortia such as the one led by the French Health Data Hub. These workstreams will mainly focus on getting an understanding on what the legislative and policy blockers are in Europe and how it could be resolved, by looking what different Member States are doing. The ultimate goal is trying to find a European approach both for primary and secondary use that would make the EHDS possible.
- Healthcare Interoperability: The EHDS has as a big focus on Interoperability and FAIR data principles. There is currently no healthcare standard defined yet, such as FHIR, openEHR or OMOP. We already see organizations such as HL7, setting up new initiatives such as the Hospitals-on-FHIR, where they want to prepare Hospitals for the European Health Data Space, with a HoF Maturity Model (HoF-MM). One particular thing that has been decided, is how metadata will be exposed in the EHDS. This will be done via DCAT Application Profile. DCAT-AP will be the baseline specification on how metadata records will be explored for semantic interoperability in the EHDS and with other European data space.
Personally, I would love to have more insights on the architectural side of the EHDS, such as how data will be transmitted across member states, will REST be used to serve and consume data? Are there already specifications on how Authentication and Authorization will be done, will there be a central Identity Provider? How will the secure processing environments be setup, could this something like the Azure TRE?
Nevertheless, I am very excited about the European Health Data Space, and the opportunities it will bring for primary and secondary use of data in Europe. There is already a lot going on to make the EHDS a reality, but there are also many challenges that will need to be uncovered, solved and agreed on by the Member States to make this reality in 2030.
Bert
Sources:
- European Patients Forum 2022 (europa.eu)
- 210617_15.00_Europe Vision 3_Natalia Zylinska.pdf (ihe-europe.net)
- tehdas-leaflet-2022.pdf
- Data Analysis and Real World Interrogation Network (DARWIN EU) | European Medicines Agency (europa.eu)
- Project – Tehdas
- Press release – EHDS2 pilot launch (health-data-hub.fr)
- Hospitals-on-FHIR: Preparing Hospitals for European Health Data Space – HealthManagement.org